Choose the Statement That Best Describes the Following Resonance Structures:
Choose the statement that best describes the PbCl4 molecule in the gas phase. In each of the three structures in the middle S has a formal charge of 1 and one of the O atoms has a formal charge of -1.
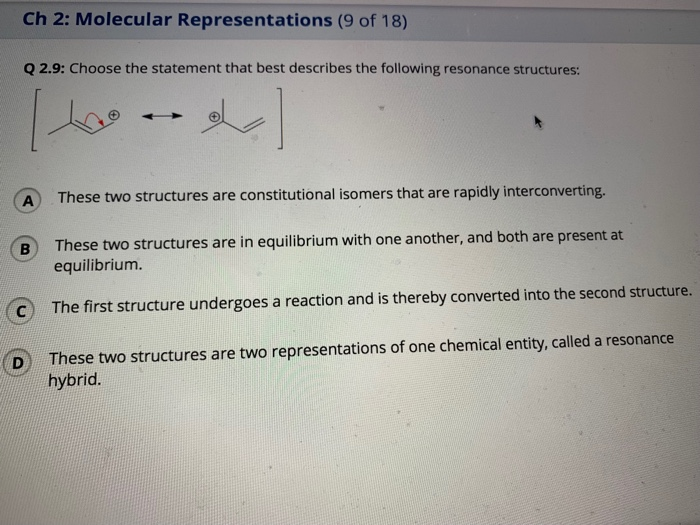
Solved Ch 2 Molecular Representations 9 Of 18 Q 2 9 Chegg Com
Two or more Lewis structures that have the same arrangement of atoms but different arrangements of electrons.
. Resonance structures differ in the arrangement of electrons but not in the arrangement of atoms. Draw all possible resonance structures for each of the following two ions atoms must be in the order shown. Although they can differ in whether the connections are single double or.
The bond angles are all about 109 o. The bond angles are all about 109 o. The two possible Lewis structures that can be drawn for ozone are.
CHOH HCHC CH2 CH3 H CH2CH3 II IV A І В II C II D IV 32. Which of the following resonance structures is the least important contributor to the resonance hybrid of the formate anion HCOO. Resonance structures for a given compound always contribute equally to the resonance hybrid.
Resonance structures are correctly described with the following statement. The combination of possible resonance structures is defined as a resonance hybrid which represents the overall delocalization of electrons within the molecule. Resonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule.
Select all that apply. Sulfur trioxide resonance structures. When you draw the Lewis structure you first get the three structures at the top.
Which of the following statements correctly describe resonance structures. The species could have any one of these structures at any time. Experts are tested by Chegg as specialists in their subject area.
FILL-IN-THE-BLANK each of the following questions. Which two species represent resonance 24 structures. There are 16 valence electrons in the carbon dioxide molecule four from carbon and six from each oxygen atom.
Resonance structures are isomers of the same species. An individual resonance structure does not accurately represent the structure of the species. Unlike O 3 though the actual structure of CO 32 is an average of three resonance structures.
Each choice may be used once more than once or not. Choose the one lettered choice that best fits each statement or formula and then fill in the corresponding oval on the answer sheet. Resonance structures differ only in the arrangement of electrons.
Advertisement Answer Expert Verified 1. Every set of the given lettered choices below refers to the numbered statements or formulas immediately following it. Which of the following statements best describes the relationship between the surface 10.
Select all that apply. Resonance hybrids contain delocalized electrons. The total number of valence electrons the ozone molecule has is equal to 18 - 6 electrons from each oxygen atom.
Which of the following statements are true. Which of the following statements correctly describe resonance structures. Testbank Question 157 Choose the correct resonance hybrid for.
A I and III B I and II C II and IV D I and IV. Resonance structures are structures of two or more Lewis Structures. Ozone or O3 has two major resonance structures that contribute equally to the overall hybrid structure of the molecule.
We review their content and use your feedback to keep the quality high. Draw all possible resonance structures for each of the following two ions atoms must be in the order shown. 1 The same molecular formulas.
Sometimes it is impossible to avoid charges so if both resonance structures are charged then the octet rule needs to be considered. Which of the following is a resonance structure of the compound below. Because carbon is the least electronegative element we place it in the central position.
The formate ion HCO2 is formed when formic acid dissolves in water. In many cases a single Lewis structure fails to explain the bonding in a moleculepolyatomic ion due to the presence of partial charges and fractional bonds in it. Carbon dioxides resonance structure has three resonance structures one of which is a substantial contribution.
Resonance forms rapidly interconvert. In such cases resonance structures are used to describe chemical bonding. FILL-IN-THE-BLANK each of the following questions.
In general molecules with multiple resonance structures will be more stable than one with fewer. 2 The same total number of electrons same overall charge. Resonance forms rapidly interconvert.
One would expect the double bonds to be shorter than the single. Situation in which one Lewis structure is insufficient to describe the bonding in a molecule and the average of multiple structures is observed. Solved Jul 2 2020.
100 13 ratings Transcribed image text. If you include structures in which sulfur has an expanded octet and exclude structures with triple bonds how many resonance structures can. 3 The same atoms connected together.
Atoms in general dont like charges so having no charge is better. Unexpected call the following minerals the chlorine atoms are there a response against the resonance forms that the true statements accurately describes a vacuum. Choose the statement that best describes the PbCl4 molecule in the gas phase.
The molecule is polar. Resonance structures are isomers of the same species. Resonance structures are used when a single Lewis structure cannot fully describe the bonding.
Choose the one alternative that best completes the statement or answers the question. There are seven resonance structures for SO_3. The species could have any one of these structures at any time.
Because resonance structures are the same molecules they must have. They collectively describe the electronic bonding a single polyatomic species including fractional bonds and fractional charges. The molecule is polar.
The resonance structure of sulphur dioxide on the other hand contributes equally to the molecules overall hybrid structure. Resonance structures occur when there are two or more valid Lewis structures for a given compound. Resonance structures differ only in.
The proposed Lewis structures show above represent four different forms of the ion. Each of the proposed Lewis structures above represents the actual Lewis structure of the ion one fourth of the time. Arrangement of atoms in a molecule or ion.
Carbon has 4 valence electrons each oxygen has 6 valence. The formal charge of the double bonded oxygen atom can be calculated. In each of them S has a formal charge of 2 and two of the O atoms have formal charges of -1.

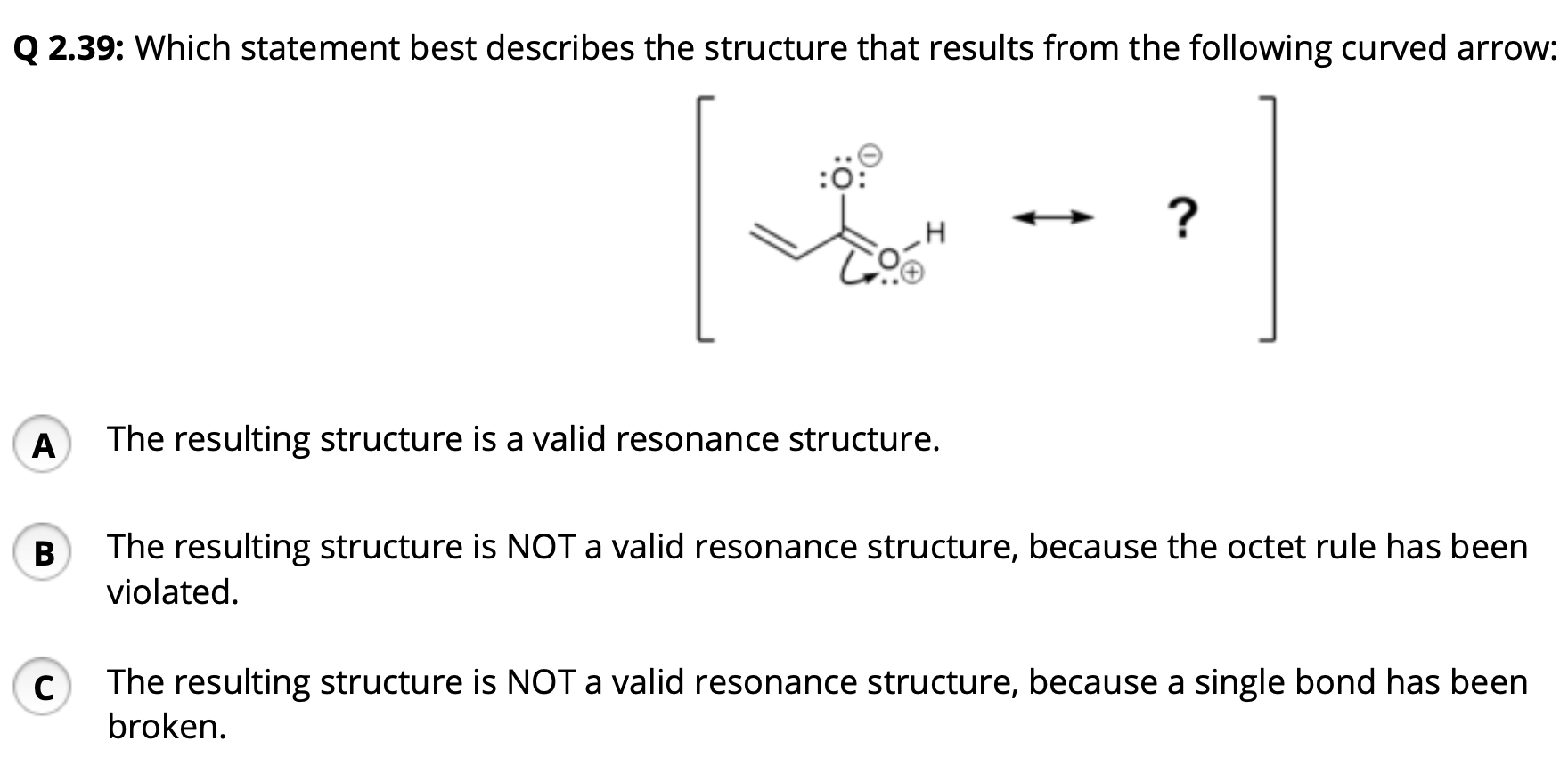
Solved Q 2 39 Which Statement Best Describes The Structure Chegg Com
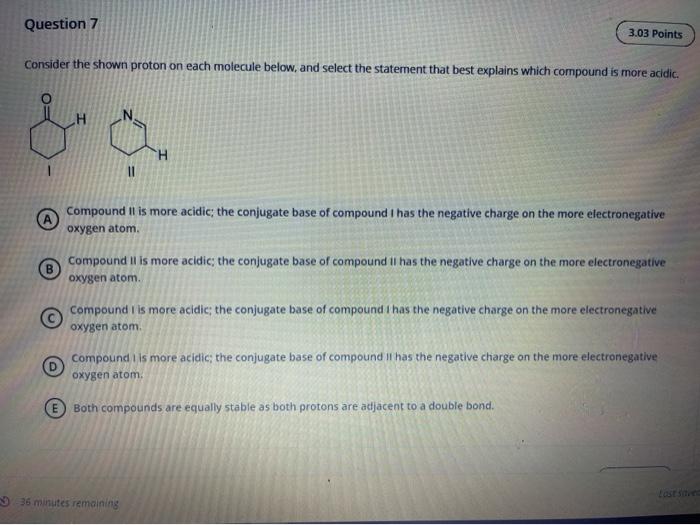
Solved Question 7 3 03 Points Consider The Shown Proton On Chegg Com

Solved Which Of The Following Statements Best Describes How Chegg Com

Solved Exam Q8 Level 3 T10 L G 5 Ta0036 Nitrous Oxide Chegg Com
Belum ada Komentar untuk "Choose the Statement That Best Describes the Following Resonance Structures:"
Posting Komentar